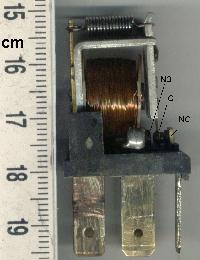
Relay
| This article needs additional citations for verification. Please help improve this article by adding reliable references. Unsourced material may be challenged and removed. (November 2009) |
A relay is an electrically operated switch. Many relays use an electromagnet to operate a switching mechanism mechanically, but other operating principles are also used. Relays are used where it is necessary to control a circuit by a low-power signal (with complete electrical isolation between control and controlled circuits), or where several circuits must be controlled by one signal. The first relays were used in long distance telegraph circuits, repeating the signal coming in from one circuit and re-transmitting it to another. Relays were used extensively in telephone exchanges and early computers to perform logical operations.
A type of relay that can handle the high power required to directly control an electric motor is called a contactor. Solid-state relays control power circuits with no moving parts, instead using a semiconductor device to perform switching. Relays with calibrated operating characteristics and sometimes multiple operating coils are used to protect electrical circuits from overload or faults; in modern electric power systems these functions are performed by digital instruments still called "protective relays".
Contents
[hide]- 1 Basic design and operation
- 2 Types
- 3 Pole and throw
- 4 Applications
- 5 Relay application considerations
- 6 Protective relays
- 7 Railway signalling
- 8 See also
- 9 References
- 10 External links
بقیه و متن کامل مقاله در ادامه مطالب...
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow (as opposed to ionic conductivity) intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter. Semiconductor materials are the foundation of modern electronics, including radio, computers, telephones, and many other devices. Such devices include transistors, solar cells, many kinds of diodes including the light-emitting diode, the silicon controlled rectifier, and digital and analog integrated circuits. Similarly, semiconductor solar photovoltaic panels directly convert light energy into electrical energy. In a metallic conductor, current is carried by the flow of electrons. In semiconductors, current is often schematized as being carried either by the flow of electrons or by the flow of positively charged "holes" in the electron structure of the material. Actually, however, in both cases only electron movements are involved.
Common semiconducting materials are crystalline solids, but amorphous and liquid semiconductors are known. These include hydrogenated amorphous silicon and mixtures of arsenic, selenium and tellurium in a variety of proportions. Such compounds share with better known semiconductors intermediate conductivity and a rapid variation of conductivity with temperature, as well as occasional negative resistance. Such disordered materials lack the rigid crystalline structure of conventional semiconductors such as silicon and are generally used in thin film structures, which are less demanding for as concerns the electronic quality of the material and thus are relatively insensitive to impurities and radiation damage. Organic semiconductors, that is, organic materials with properties resembling conventional semiconductors, are also known.
Silicon is used to create most semiconductors commercially. Dozens of other materials are used, including germanium, gallium arsenide, and silicon carbide. A pure semiconductor is often called an “intrinsic” semiconductor. The electronic properties and the conductivity of a semiconductor can be changed in a controlled manner by adding very small quantities of other elements, called “dopants”, to the intrinsic material. In crystalline silicon typically this is achieved by adding impurities of boron or phosphorus to the melt and then allowing the melt to solidify into the crystal. This process is called "doping".[1]
Contents
[hide]- 1 Explaining semiconductor energy bands
- 2 Energy bands and electrical conduction
- 3 Holes: electron absence as a charge carrier
- 4 Energy–momentum dispersion
- 5 Carrier generation and recombination
- 6 Semi-insulators
- 7 Doping
- 8 Preparation of semiconductor materials
- 9 See also
- 10 References
- 11 Further reading
- 12 External links
بقیه و متن کامل مقاله در ادامه مطالب....
Electronics

Electronics is the branch of science and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits. The nonlinear behaviour of these components and their ability to control electron flows makes amplification of weak signals possible, and is usually applied to information and signal processing. Electronics is distinct from electrical and electro-mechanical science and technology, which deals with the generation, distribution, switching, storage and conversion of electrical energy to and from other energy forms using wires, motors, generators, batteries, switches, relays, transformers, resistors and other passive components. This distinction started around 1906 with the invention by Lee De Forest of the triode, which made electrical amplification of weak radio signals and audio signals possible with a non-mechanical device. Until 1950 this field was called "radio technology" because its principal application was the design and theory of radio transmitters, receivers and vacuum tubes.
Today, most electronic devices use semiconductor components to perform electron control. The study of semiconductor devices and related technology is considered a branch of solid state physics, whereas the design and construction of electronic circuits to solve practical problems come under electronics engineering. This article focuses on engineering aspects of electronics.
Contents
[hide]- 1 Electronic devices and components
- 2 Types of circuits
- 3 Heat dissipation and thermal management
- 4 Noise
- 5 Electronics theory
- 6 Electronics lab
- 7 Computer aided design (CAD)
- 8 Construction methods
- 9 See also
- 10 References
- 11 Further reading
- 12 External links
بقیه و متن کامل مقاله در ادامه مطالب...
Transistor
A transistor is a semiconductor device used to amplify and switch electronic signals. It is made of a solid piece of semiconductor material, with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals changes the current flowing through another pair of terminals. Because the controlled (output) power can be much more than the controlling (input) power, the transistor provides amplification of a signal. Today, some transistors are packaged individually, but many more are found embedded in integrated circuits.
The transistor is the fundamental building block of modern electronic devices, and is ubiquitous in modern electronic systems. Following its release in the early 1950s the transistor revolutionized the field of electronics, and paved the way for smaller and cheaper radios, calculators, and computers, among other things.
Contents
[hide]- 1 History
- 2 Importance
- 3 Simplified operation
- 4 Comparison with vacuum tubes
- 5 Types
- 6 Construction
- 7 See also
- 8 References
- 9 Further reading
- 10 External links
بقیه و متن کامل مقاله در ادامه مطالب...
Electronic band structure
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and Bravais lattice. The band structure of a material determines several characteristics, in particular the material's electronic and optical properties.
Contents
[hide]- 1 Why bands occur in materials
- 2 Basic concepts
- 3 Theory of band structures in crystals
- 4 References
- 5 Bibliography
- 6 Further reading
- 7 See also
بقیه و متن کامل مقاله در ادامه مطالب....
Electron
 |
|
| Composition: | Elementary particle[2] |
| Particle statistics: | Fermionic |
| Group: | Lepton |
| Generation: | First |
| Interaction: | Gravity, Electromagnetic, Weak |
| Symbol(s): | e− , β− |
| Antiparticle: | Positron (also called antielectron) |
| Theorized: | Richard Laming (1838–1851),[3] G. Johnstone Stoney (1874) and others.[4][5] |
| Discovered: | J. J. Thomson (1897)[6] |
| Mass: | 9.10938215(45)×10−31 kg[7] 5.4857990943(23)×10−4 u[7] [1,822.88850204(77)]−1 u[note 1] 0.510998910(13) MeV/c2[7] |
| Electric charge: | −1 e[note 2] −1.602176487(40)×10−19 C[7] −4.803×10−10 esu [8] |
| Magnetic moment: | −1.00115965218111 μB[7] |
| Spin: | 1⁄2 |
The electron is a subatomic particle carrying a negative electric charge. It has no known components or substructure. Therefore, the electron is generally believed to be an elementary particle.[2] An electron has a mass that is approximately 1/1836 that of the proton[9] The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion. The antiparticle of the electron is called the positron. The positron is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may either scatter off each other or be totally annihilated, producing a pair (or more) of gamma ray photons. Electrons, which belong to the first generation of the lepton particle family,[10] participate in gravitational, electromagnetic and weak interactions.[11] Electrons, like all matter, have quantum mechanical properties of both particles and waves, so they can collide with other particles and be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[10]
The concept of an indivisible amount of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopher Richard Laming;[4] the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.[6][12][13]
In many physical phenomena, such as electricity, magnetism, and thermal conductivity, electrons play an essential role. An electron in motion relative to an observer generates a magnetic field, and will be deflected by external magnetic fields. When an electron is accelerated, it can absorb or radiate energy in the form of photons. Electrons, together with atomic nuclei made of protons and neutrons, make up atoms. However, electrons contribute less than 0.06% to an atom's total mass. The attractive Coulomb force between an electron and a proton causes electrons to be bound into atoms. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[14]
According to theory, most electrons in the universe were created in the big bang, but they may also be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. Electrons may be destroyed through annihilation with positrons, and may be absorbed during nucleosynthesis in stars. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including welding, cathode ray tubes, electron microscopes, radiation therapy, lasers and particle accelerators.
Contents
[hide]- 1 History
- 2 Characteristics
- 3 Formation
- 4 Observation
- 5 Plasma applications
- 6 See also
- 7 Notes
- 8 References
- 9 External links
بقیه و متن کامل مقاله در ادامه مطالب....
Amplitude is the magnitude of change in the oscillating variable with each oscillation within an oscillating system. For example, sound waves in air are oscillations in atmospheric pressure and their amplitudes are proportional to the change in pressure during one oscillation. If a variable undergoes regular oscillations, and a graph of the system is drawn with the oscillating variable as the vertical axis and time as the horizontal axis, the amplitude is visually represented by the vertical distance between the extrema of the curve.
In older texts the phase is sometimes very confusingly called the amplitude.[1]
Contents
[hide]
بقیه و متن کامل مقاله در ادامه مطالب....
Frequency

Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency. The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency. Loosely speaking, 1 year is the period of the Earth's orbit around the Sun,[1] and the Earth's rotation on its axis has a frequency of 1 rotation per day.[2]
Contents
[hide]- 1 Definitions and units
- 2 Measurement
- 3 Frequency of waves
- 4 Examples
- 5 Period versus frequency
- 6 Other types of frequency
- 7 Frequency ranges
- 8 See also
- 9 References
- 10 Further reading
- 11 External links
بقیه و متن کامل مقاله در ادامه مطالب....
Radio is the transmission of signals by modulation of electromagnetic waves with frequencies below those of visible light.[1] Electromagnetic radiation travels by means of oscillating electromagnetic fields that pass through the air and the vacuum of space. Information is carried by systematically changing (modulating) some property of the radiated waves, such as amplitude, frequency, phase, or pulse width. When radio waves pass an electrical conductor, the oscillating fields induce an alternating current in the conductor. This can be detected and transformed into sound or other signals that carry information.
Contents
[hide]- 1 Etymology
- 2 Processes
- 3 History
- 4 Uses of radio
- 5 See also
- 6 References
- 7 Further reading
- 8 External links
بقیه و کامل مقاله در ادامه مطالب...
Radar


Radar is an object-detection system which uses electromagnetic waves—specifically radio waves—to determine the range, altitude, direction, or speed of both moving and fixed objects such as aircraft, ships, spacecraft, guided missiles, motor vehicles, weather formations, and terrain. The radar dish, or antenna, transmits pulses of radio waves or microwaves which bounce off any object in their path. The object returns a tiny part of the wave's energy to a dish or antenna which is usually located at the same site as the transmitter.
Practical radar was developed in secrecy during World War II by Britain and other nations. The term RADAR was coined in 1940 by the U.S. Navy as an acronym for radio detection and ranging.[1][2] The term radar has since entered the English and other languages as the common noun radar, losing all capitalization. In the United Kingdom, the technology was initially called RDF (range and direction finding), using the same initials used for radio direction finding to conceal its ranging capability[citation needed].
The modern uses of radar are highly diverse, including air traffic control, radar astronomy, air-defense systems, antimissile systems; nautical radars to locate landmarks and other ships; aircraft anticollision systems; ocean-surveillance systems, outer-space surveillance and rendezvous systems; meteorological precipitation monitoring; altimetry and flight-control systems; guided-missile target-locating systems; and ground-penetrating radar geological observations.
Other systems similar to radar have been used in other parts of the electromagnetic spectrum. One example is "lidar", which uses visible light from lasers rather than radio waves.
بقیه و کامل مقاله در ادامه مطالب.....